
Drlogy
Healthcare organization
Pathology Report Format: 14 Guide For Making Lab Report Format
Hello lab owners, looking to stand out from the crowd? Your pathology report format is your secret weapon. At Drlogy, we've got your back with a guide that'll make your reports shine. With our help, you'll build trust with patients and doctors alike, showing them you mean business when it comes to accuracy and quality.
Recommended
Unlock the 14-step guide to mastering patient attraction from A to Z with Drlogy Pathology Report Format as per guidelines set by ICMR (The Indian Council of Medical Research). Elevate your practice, captivate patients, and dominate your market effortlessly. Let's Start!
What is Pathology Report Format?
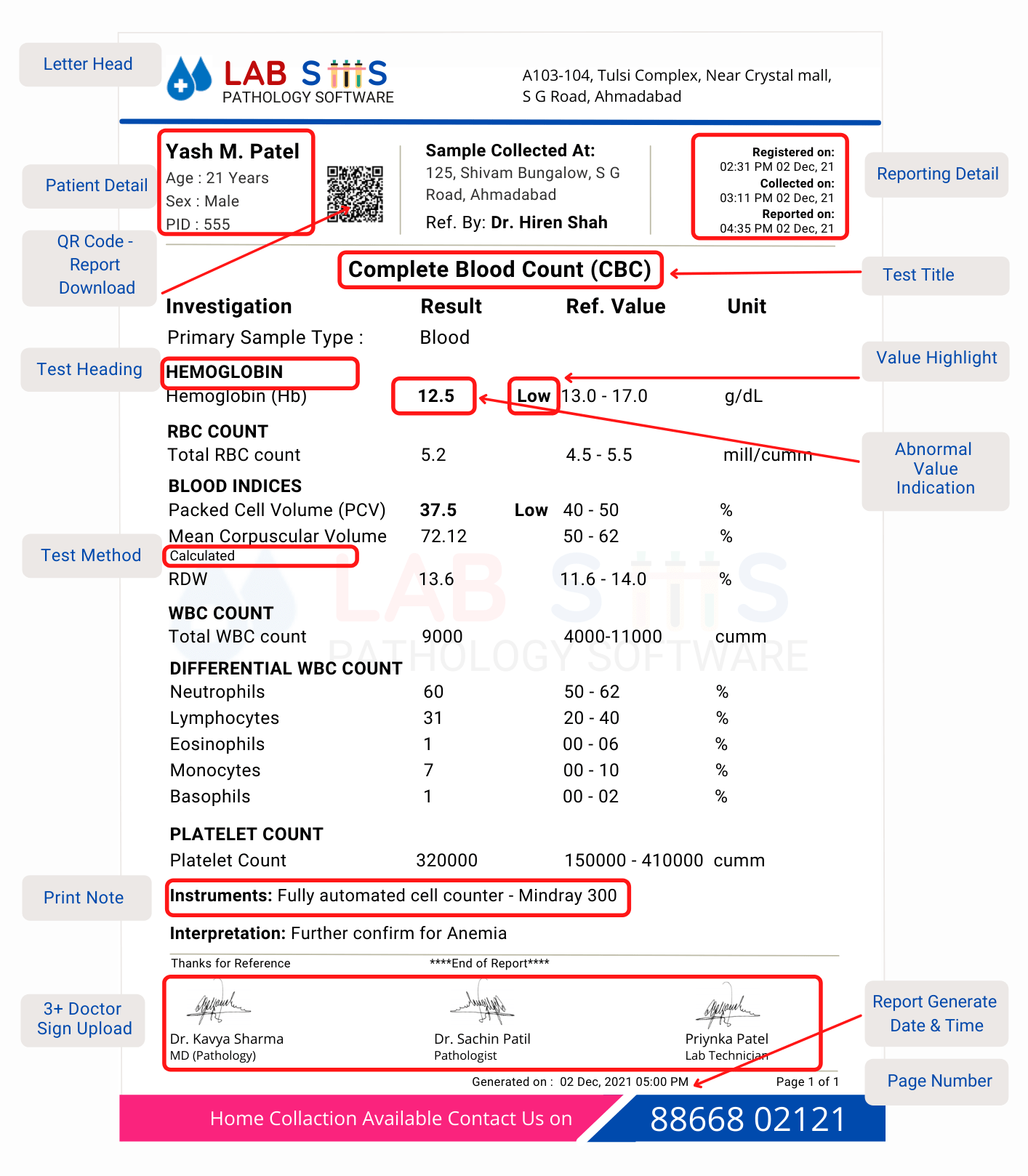
Standard pathology report format typically includes proper standard structures which should have the following information in your lab reports.
| Patient Details | Main Test Body Format |
| Turnaround Time (TAT) Info | Registration Number |
| Referring Doctor's Name | Sample Details |
| Results | Interpretation |
| Lab Information | Credentials |
| Lab In-Charge and Pathologist Signatures | Page Numbers |
- Pathology report format outlines the structure and organization of diagnostic reports generated by pathology laboratories.
- It includes sections for patient information, test details, results, interpretation, and signatures for authentication.
- The format ensures clarity, consistency, and accuracy in reporting, aiding in effective communication between pathologists, clinicians, and patients.
14 Steps Guide Foreal Lab Pathology Report Format
Here are 14 Steps Guide Foreal Lab Pathology Report Format.
1. Header Format:
- Include the name and logo of the laboratory for brand recognition.
- Ensure clarity and professionalism in font choice and layout.
- Incorporate relevant contact information, such as address and phone number.
- Maintain consistency in header placement across all reports.
- Consider adding the date of report generation for reference.
2. Footer Format:
- Place the footer at the bottom of each page for continuity.
- Include essential legal disclaimers or privacy statements.
- Optionally, provide additional contact information for inquiries.
- Ensure that the footer does not interfere with readability or aesthetics.
- Consider including a page number for easy navigation.
3. Patient Information:
- Collect patient details including name, age, gender, and any uniqueentifiers.
- Verify patient information for accuracy and completeness.
- Include relevant medical history or clinical information if available.
- Respect patient confidentiality and adhere to HIPAA guidelines.
- Ensure clear and consistent formatting of patient information across reports.
4. Test Body Format:
- Organize test information systematically, starting with the test name and code.
- Include details on specimen collection, preparation, and analysis methods.
- Present results in a structured and easy-to-read format, such as tables or graphs.
- Provide units of measurement and reference ranges for each test parameter.
- Highlight any abnormal findings or critical values for immediate attention.
5. Result & Ref Ranges:
- Clearly present test results alongside corresponding reference ranges.
- Use appropriate terminology and abbreviations for clarity.
- Differentiate between normal and abnormal results for easy interpretation.
- Ensure consistency in formatting to enhance readability.
- Provide context or clinical implications for abnormal findings when necessary.
6. Interpretation & Instrument:
- Offer clinical interpretations of test results, considering the patient's overall health status.
- Discuss the significance of results in the context of the diagnostic process.
- Include information on the instrument or methodology used for testing.
- Highlight any limitations or caveats in the interpretation process.
- Encourage collaboration between pathologists and clinicians for accurate diagnosis.
7. Lab Info & Letterhead:
- Display laboratory accreditation, certification, or accreditation logos prominently.
- Incorporate the laboratory's official letterhead for authenticity.
- Include relevant information about the laboratory's location, accreditation status, and contact details.
- Ensure that the letterhead design aligns with the laboratory's branding guidelines.
- Provide links or QR codes for additional information about the laboratory's services or accreditation.
8. Signature:
- Require signatures from both the lab in-charge and the pathologist for report authentication.
- Ensure that signatures are legible and accompanied by printed names and credentials.
- Implement electronic signature solutions for digital reports.
- Include a timestamp or date of signature for record-keeping purposes.
- Establish protocols for signature verification and authorization.
9. Additional Notes:
- Allow space for clinicians to add supplementary notes or comments.
- Encourage communication between pathologists and referring physicians through note sections.
- Provide guidelines for the type and content of additional notes.
- Ensure that additional notes do not obscure critical information in the report.
- Consider using standardized templates or prompts for consistency in note-taking.
10. Quality & Simplicity:
- Emphasize accuracy, reliability, and quality assurance in report generation.
- Strive for simplicity and clarity inuage and presentation.
- Implement quality control measures to minimize errors and discrepancies.
- Regularly review and update report formats to reflect best practices and advancements.
- Solicit feedback from users toentify areas for improvement in report quality and simplicity.
11. Mistakes To Avoid:
- Train staff to recognize and avoid common errors in report generation.
- Implement double-check procedures for critical data entry points.
- Provide ongoing education and training on best practices in pathology reporting.
- Regularly audit reports for errors or inconsistencies.
- Establish protocols for addressing and correcting mistakes in reports.
12. Report Templates:
- Develop standardized report templates for consistency and efficiency.
- Customize templates to accommodate different types of tests or specialties.
- Include placeholders for patient information, test results, and interpretations.
- Ensure that templates adhere to regulatory requirements and accreditation standards.
- Provide training and guidelines for staff on template utilization and customization.
13.eal Report Format:
- Facilitates personalized report sharing communication for patient understanding and engagement.
- Ensures secure and official delivery of reports for compliance and confidentiality.
- Provides quick and convenient access to report summaries for immediate awareness.
- Enables efficient electronic delivery and archiving of reports for easy retrieval.
- Offers instant and accessible communication for report sharing, enhancing patient convenience.
14. LIMS:
- Implement a Laboratory Information Management System (LIMS) for efficient report generation and management.
- Utilize LIMS functionalities for sample tracking, result entry, and data analysis.
- Integrate LIMS with other laboratory systems for seamless data exchange.
- Ensure data security and compliance with regulatory requirements in LIMS implementation.
- Provide training and support for staff to maximize LIMS utilization and efficiency.
Pathology Report Format Guide
Here are 14 steps guide for designing a pathology lab report format from A to Z.
Summary
Overall, By using this series learn to structure pathology reports effectively, ensuring clear presentation of patient information, test results, and interpretations, while maintaining consistency and professionalism throughout. Master the utilization of standardized formats and incorporate modern trends like QR codes for enhanced report authenticity and user confidence.
Check Drlogy Pathology Report Format Guide For A to Z information regarding how to make proper report format structure to implementation of report format into you lab work.




